Fill in missing sub- and superscripts for all particles to complete the following equation for beta decay. 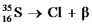
Correct Answer:
Verified
Q39: The isotope Q40: A certain isotope has a specific Q52: The radiochemist, Will I. Glow, studied Q63: Write a complete, balanced equation to represent Q64: Exposure to 10 nCi for 10 minutes Q67: Explain how the number of protons and Q72: Write a complete, balanced equation to represent Q74: Calcium-39 undergoes positron decay. Each positron carries Q77: Sodium-21 will emit positrons each having an Q80: Write a complete, balanced equation to represent![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents