A bottle of vintage red wine has lost its label.The concentration of tritium ( 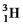
)in the wine is 0.34 times that found in freshly bottled wines.If the half-life of tritium is 12.3 years,estimate the time elapsed since the wine was bottled.
Correct Answer:
Verified
Q3: Radioactive decay follows zero-order kinetics.
Q8: Most foodstuffs contain natural, radioactive isotopes.
Q15: Positron decay and electron capture have the
Q16: Gamma rays are not deflected by an
Q17: Gamma rays are high energy electrons.
Q65: When an electron and its anti-particle,a
Q68: The binding energy per nucleon reaches a
Q70: Briefly, explain the relationship between the rad
Q75: Bombardment of uranium-238 nuclei by carbon-12 nuclei
Q85: What is the mechanism by which control
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents