The masses of a potassium-40 atom,a proton and a neutron are 39.963999 amu,1.007825 amu and 1.008665 amu,respectively.Calculate to four significant figures
a.the mass defect in amu and
b.the energy released in MeV/nucleon,in the formation of 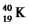
from the appropriate number of protons and neutrons.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: No alpha decay is observed for isotopes
Q3: Radioactive decay follows zero-order kinetics.
Q5: After 4 half-lives, the fraction of a
Q8: Most foodstuffs contain natural, radioactive isotopes.
Q59: Fill in missing sub- and superscripts for
Q61: Calculate to four significant figures
a.the mass
Q65: When an electron and its anti-particle,a
Q68: The binding energy per nucleon reaches a
Q73: Write a complete, balanced equation to represent
Q75: Bombardment of uranium-238 nuclei by carbon-12 nuclei
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents