Which of the following statements is false?
A) The limiting reactant is completely consumed in a chemical reaction.
B) The theoretical yield is the amount of product that can be made based on the amount of limiting reagent.
C) The actual yield is the amount of product actually produced by a chemical reaction.
D) The percent yield = 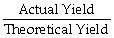
× 100%
E) All of the above are true statements.
Correct Answer:
Verified
Q45: Given that 4 NH3 + 5 O2
Q46: How many grams of sodium metal are
Q47: How many moles of water are made
Q48: How many moles of aluminum are needed
Q49: How many moles of chlorine gas are
Q52: Given the balanced equation CH4 + 2
Q57: How many moles of water are made
Q60: How many moles of H2 can be
Q63: Which ingredient is the limiting reactant if
Q78: A chemist wishes to perform the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents