Increasing the volume of the system below causes the reaction to shift towards the right. 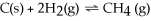
Correct Answer:
Verified
Q22: If Keq = 2 for the reaction
Q27: Adding heat to an endothermic reaction causes
Q30: Decreasing the amount of carbon dioxide in
Q30: Le Chatelier's principle states that when a
Q34: Le Chatelier's principle states that a chemical
Q34: A compound with a very large Ksp
Q35: The equilibrium expression for the reaction:
Q36: Decreasing the volume of the system below
Q37: If the equilibrium constant,Keq,for a reaction increases
Q48: If an equilibrium reaction shifts to the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents