Dissolving the compound PbCl2 into water can be represented as 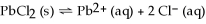
,
so the equilibrium expression is Ksp = 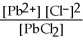
.
Correct Answer:
Verified
Q34: A compound with a very large Ksp
Q35: The equilibrium expression for the reaction:
Q36: Decreasing the volume of the system below
Q37: If the equilibrium constant,Keq,for a reaction increases
Q38: A compound with a relatively small Ksp
Q40: Increasing the amount of carbon in the
Q45: Why does the rate of the reaction
Q46: A chemical equilibrium exists when:
A)reactants are completely
Q53: A system is said to be in
Q56: A description of life is:
A)Living things maintain
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents