For the reaction aA + bB ⇌ cC + dD,the equilibrium expression is:
A) Keq = 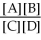
B) Keq = 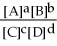
C) Keq = 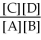
D) Keq = 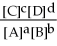
E) none of the above
Correct Answer:
Verified
Q45: Which equilibrium constant represents a reaction that
Q45: Why does the rate of the reaction
Q46: Which of the following is TRUE of
Q46: A chemical equilibrium exists when:
A)reactants are completely
Q50: Which of the following changes will increase
Q50: For the reaction 2A + B ⇌
Q52: The chemical equation that would generate the
Q53: Which of the following is TRUE of
Q59: Suppose a wall divides a playground,and twenty
Q60: Which of the following changes will increase
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents