Ca2+ (aq)+ 2Na (s)→ Ca (s)+ 2 Na+ (aq)is a spontaneous reaction.
Activity Series = 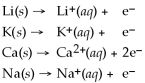
Correct Answer:
Verified
Q24: Electrical current can be used to drive
Q31: A galvanic cell is a spontaneous electrochemical
Q35: Electrolysis is used to recover many metals
Q37: To achieve the largest battery voltage possible,the
Q38: Flashlight batteries are called dry cells.
Q41: In the following reaction,
Mg (s)+ Cu2+ (aq)→
Q42: For the reaction Si (s)+ O2 (g)→
Q46: Which of the following are typically TRUE
Q50: Which fact about fuel cells is FALSE?
A)Fuel
Q54: The reducing agent typically:
A)gains electrons.
B)always remains unchanged
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents