Diatomic N2 can react with diatomic H2 to form ammonia (NH3) .The balanced chemical equation is: 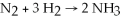
If 6 moles of H2 totally reacted with more than enough N2,how many moles of ammonia would be expected to form?
A) 2 moles
B) 3 moles
C) 4 moles
D) 6 moles
E) not enough information
Correct Answer:
Verified
Q43: How many grams of water are made
Q46: How many grams of sodium metal are
Q48: How many moles of aluminum are needed
Q49: How many moles of chlorine gas are
Q52: How many grams of water are made
Q53: How many eggs are needed to make
Q54: Given that 4 NH3 + 5 O2
Q56: Many metals react with halogens to give
Q57: How many moles of water are made
Q60: How many moles of H2 can be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents