A balanced chemical equation used to prepare ammonium carbonate, (NH4) 2CO3 ,is: 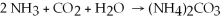
Which choice of reactant quantities shown below would result in the greatest amount of ammonium carbonate being formed?
A) React 2 moles NH3 ,1 mole CO2 ,and 1 mole H2O
B) React 2 moles NH3 ,8 moles CO2 ,and 8 moles H2O
C) React 4 moles NH3 ,1 mole CO2 ,and 2 moles H2O
D) React 4 moles NH3 ,2 moles CO2 ,and 2 moles H2O
E) none of the above
Correct Answer:
Verified
Q63: Which ingredient is the limiting reactant if
Q64: How many grams of NO2 are theoretically
Q68: What is the excess reactant for the
Q70: What is the theoretical yield of waffles
Q71: A tricycle factory uses the following items
Q71: Which of the following statements is false?
A)The
Q72: Determine the theoretical yield of C when
Q76: Consider the following generic chemical equation: 2W
Q79: How many grams of calcium phosphate are
Q80: How many moles of lithium nitrate are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents