What is the final volume of a 500.0 mL gas container that increased in temperature from 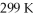
To 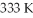
While the pressure increased from 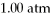
To 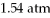
?
A) 0.691 L
B) 2.77 L
C) 0.362 L
D) 1.45 L
E) none of the above
Correct Answer:
Verified
Q63: Suppose a balloon was released from the
Q64: What is the initial temperature (°C)of a
Q65: Gas density can be calculated by dividing
Q67: What is the final volume of a
Q68: A balloon originally had a volume of
Q69: What is the initial temperature of a
Q70: If the initial pressure of a system
Q72: Which of the following statements is TRUE
Q76: If the volume of a gas container
Q80: A certain volume of gas was confined
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents