Which of the following diatomic elements would have a mass of 19.08 grams stored in a 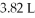
Container at 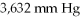
And 100°C? (R= 0.0821 L atm/ mol K)
A) 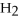
B) 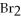
C) 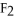
D) 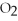
E) not enough information.
Correct Answer:
Verified
Q81: What is the major component of the
Q83: A "shielding gas" mixture of argon and
Q85: A sample of 0.255 mole of gas
Q87: Avogadro's Law is expressed as:
A)V is proportional
Q89: To solve problems using Avogadro's Law,which mathematical
Q91: What is the pressure of a 3.00
Q92: If each of the following gas samples
Q96: Which of the following gas law relationships
Q97: What problem could happen if deep sea
Q98: What is the third most abundant component
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents