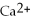
(aq)+ 2Na (s)→ Ca (s)+ 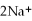
(aq)is a spontaneous reaction.
Activity Series = 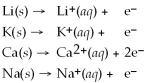
Correct Answer:
Verified
Q21: Electrons flowing through a wire is an
Q22: Given that the Activity Series shown below
Q24: Electrical current can be used to drive
Q29: Most acids dissolve metals by the oxidation
Q31: A galvanic cell is a spontaneous electrochemical
Q33: A spontaneous redox reaction can be used
Q34: An electrochemical cell is based on the
Q35: Electrolysis is used to recover many metals
Q36: Corrosion of metals may be prevented by
Q39: An electrolytic cell is a spontaneous redox
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents