Balance the redox reaction in acid solution: 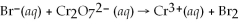
(l)
A) 4 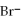
+
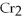
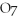
2- + 7
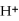
→ 2
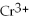
+ 2
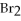
+ 7
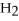
O
B) 6 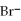
+
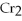
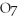
2- + 14
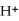
→ 2
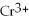
+ 3
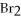
+ 7
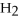
O
C) 2 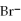
+
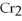
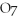
2- + 14
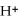
→ 2
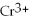
+
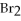
+ 7
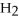
O
D) 2 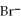
+ 2
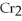
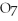
2- + 14
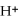
→ 4
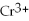
+
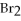
+ 14
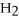
O
E) none of the above
Correct Answer:
Verified
Q88: If you properly balance the following half
Q89: From the activity list included in this
Q91: From the activity list included in this
Q92: What is the balanced oxidation half-reaction for
Q94: From the activity list included in this
Q95: From the activity list included in this
Q96: What is the balanced reduction half-reaction for
Q97: From the activity list included in this
Q98: If you properly balance the following half
Q98: From the activity list included in this
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents