Urea is a water-soluble product of nitrogen metabolism.How many hydrogen bonds can one urea molecule donate to surrounding water molecules? 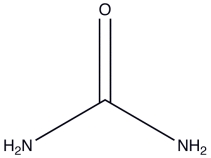
A) 2
B) 3
C) 4
D) 5
E) 6
Correct Answer:
Verified
Q5: Rank the following interactions in order of
Q6: Hydrogen bonds within liquid water
A)are attractions between
Q7: The strongest noncovalent interactions are
A)ionic interactions.
B)hydrogen bonds.
C)dipole-dipole
Q8: Matching
-The insolubility of nonpolar molecules in water
Q9: Methanol can act both as a H-bond
Q11: Which of the following statements about water
Q12: Matching
-For the _ represented by D-H
Q13: Matching
-The 104.5° bond angle in the water
Q14: Matching
-A solution containing a weak acid (pK
Q15: Which of the following statements about water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents