The following image shows two molecules of sulfur dioxide. 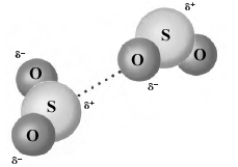 Which of the following describes the type of bonding present?
Which of the following describes the type of bonding present?
A) ionic bonding
B) polar covalent bonding
C) nonpolar covalent bonding
D) hydrogen bonding
Correct Answer:
Verified
Q65: Which of the following molecules might have
Q66: How much heat is required to melt
Q67: Phosphorus has 3 valence electrons.
Q68: What is the solvent in a solution
Q69: Consider the following two Lewis dot structures
Q71: Which of the following is a nonpolar
Q72: Choose the statement that best explains why
Q73: Consider the image below which shows a
Q74: What is the molecular geometry of carbon
Q75: Negative ions are approximately the same size
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents