Consider the following energy profile for a reaction. 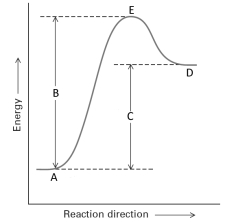 Answer with the following questions with the letters shown in the profile.
Answer with the following questions with the letters shown in the profile.
-If a catalyst were added to this reaction, the position of letter ______ would appear lower on the energy scale.
Correct Answer:
Verified
Q44: An indicator, HIn, shows a color change
Q45: Consider the following energy profile for a
Q46: Addition of a catalyst to a reaction
Q47: In the following reaction, the products have
Q48: The molar mass of sulfur trioxide is
Q49: The following energy profile represents an
exergonic reaction.
Q51: The mass of 1 mol of Ar
Q52: Consider the following energy profile for a
Q53: For a reaction to take place, the
Q54: Consider the following energy profile for a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents