Consider the following image, showing on the atomic level, the reaction occurring when a Zn wire is placed in a copper(II) nitrate solution. 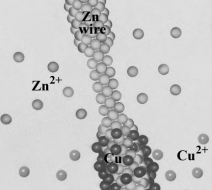 Which of the following correctly describes the image?
Which of the following correctly describes the image?
A) Zinc is being oxidized.
B) Copper is being reduced.
C) Zinc is the reducing agent.
D) All of the above are correct descriptions.
Correct Answer:
Verified
Q20: Which of the batteries listed below is
Q21: Free radicals which are also oxidizing agents
Q22: Which of the following are paired correctly?
A)
Q23: Which of the following is the function
Q24: Which of the following is a free
Q26: The reaction(s) found in primary batteries are
Q27: Which molecule would be a good oxidizing
Q28: H2 is a often oxidized and O2
Q29: Electrolysis
A) is the process of producing electricity.
B)
Q30: Vitamin C and beta-carotene are antioxidants found
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents