Consider the Following Reaction 3Fe(s) + 2Al3+(aq)
This Reaction Takes Place in the Electrochemical
Consider the following reaction.
3Fe2+(aq) + 2Al(s) 3Fe(s) + 2Al3+(aq)
This reaction takes place in the electrochemical cell shown below. 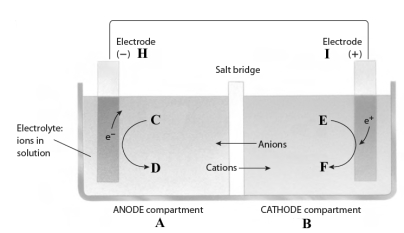
Complete the following questions using the letters shown in the image.
-The electrons flow from electrode _____ to electrode _____.
Correct Answer:
Verified
Q37: Reducing agents gain electrons and are oxidized.
Q38: The substance oxidized in an oxidation reduction
Q39: Identify the reduced species in the
Q40: Oxidation always occurs
A) with the loss of
Q41: Consider the following reaction.
3Fe2+(aq) + 2Al(s)
Q42: The reducing agent in the following
Q43: Consider the following reaction.
3Fe2+(aq) + 2Al(s)
Q45: Consider the following reaction.
3Fe2+(aq) + 2Al(s)
Q46: Consider the following reaction.
3Fe2+(aq) + 2Al(s)
Q47: Consider the following reaction.
3Fe2+(aq) + 2Al(s)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents