Consider the following model of ethanol, CH3CH2OH. 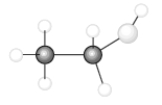 During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
During the complete combustion of ethanol with oxygen, carbon dioxide and water are formed.
-During the combustion of one mole of ethanol, the number of O-H bonds formed is _____.
Correct Answer:
Verified
Q47: Consider the following model of ethanol, CH3CH2OH.
Q48: Consider the following model of ethanol, CH3CH2OH.
Q49: Which of the bond types shown below
Q50: Which hydrocarbon would be isolated at the
Q51: The following compound could represent the principle
Q52: Identify the alkene shown below.
A) C5H12
B) C10H20
C)
Q53: The hydrocarbon represented by the model below
Q54: The correct name of the following compound
Q55: The hydrocarbon model shown below could represent
Q56: Among the alkanes, alkenes, and alkynes only
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents