Look at the bond energies O-H,N-H,and P-H in the table below.O-H is the hardest bond to break because it has the 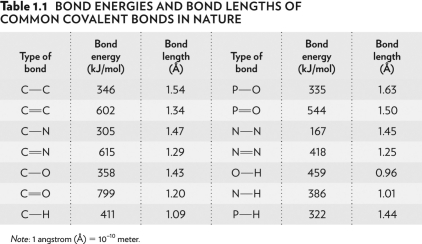
A) greatest difference in relative affinities of the two atoms for electrons.
B) smallest difference in relative affinities of the two atoms for electrons.
C) smallest difference in atomic size.
D) largest difference in atomic size.
Correct Answer:
Verified
Q8: What is the function of the chloroplast
Q9: Amino acids are the building blocks for
Q10: The birth of modern biochemistry can be
Q11: Humans do not have the enzyme cellulase.Is
Q12: Enzymes function as reaction catalysts in cells.If
Q14: Why are fewer polypeptide sequences encountered biologically
Q15: If the concentration of aspartate in the
Q16: Even though amylose and cellulose contain the
Q17: If energy in the form of ATP
Q18: The correct definition of a pathway intermediate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents