Given a biological system at 1 atm with 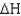 = 16 kJ/g,what is the internal energy of the system?
= 16 kJ/g,what is the internal energy of the system?
A) 15 kJ/g
B) 16 kJ/g
C) 14 kJ/g
D) Not enough information is given to calculate the answer.
Correct Answer:
Verified
Q4: For a reaction to be spontaneous,the change
Q5: Given 80 grams of water,how many calories
Q6: What is the final molecule made from
Q7: Which of the following best describes an
Q8: Energy conversion in living systems is required
Q10: The combustion of gasoline is considered exothermic
Q11: Gibbs free energy can best be defined
Q12: The difference between an oxidation reaction and
Q13: The example of water freezing into ice
Q14: Which of the following correctly describes the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents