What is the concentration of OH- in a solution that contains 3.9 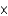 10-4 M H+?
10-4 M H+?
A) 2.6 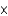 10-11 M
10-11 M
B) 3.9 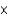 10-4
10-4
C) 2.7 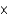 10-2 M
10-2 M
D) 1.0 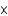 10-14 M
10-14 M
Correct Answer:
Verified
Q52: Calculate the pH of a solution that
Q53: The effects of solutes on the colligative
Q54: Calculate the concentration of pH of a
Q55: Which of the following is true?
A) A
Q56: Freezing point depression,boiling point elevation,and osmotic pressure
Q58: Osmosis occurs when water diffuses through a
A)
Q59: Hydrophobic interactions between nonpolar molecules result from
Q60: As a protein folds,what are the stabilizing
Q61: A molecule with hydrophobic and hydrophilic properties
Q62: A solution of which of the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents