Consider the reaction coordinate diagram shown below for the conversion of substrate (S)to product (P).Assume that a hypothetical enzyme is added that destabilizes the substrate (S)so that it has the free energy of S*,but otherwise the reaction is the same (in other words,it still goes through the transition state indicated in the diagram).Justify why the hypothetical enzyme is a catalyst. 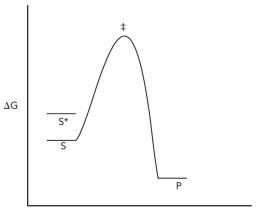
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q90: A patient presents with symptoms associated with
Q91: Explain the two ways that catalytic efficiency
Q92: What is Henry Eyring's transition state theory?
Q93: Consider the reaction coordinate diagram shown below
Q94: Propose an experiment to determine the presence
Q96: Procathepsin B is a lysosomal protease that
Q97: Given the following data for lactate dehydrogenase,calculate
Q98: Phosphorylation of _ in glycogen phosphorylase shifts
Q99: Hexokinase catalyzes the first step of glycolysis,which
Q100: What is the appropriate order of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents