For the following reaction A B,if at equilibrium 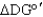 > 0,what can be said about the directionality of the reaction?
> 0,what can be said about the directionality of the reaction?
A) strongly favored in the forward direction
B) strongly favored in the reverse direction
C) strongly favored in both directions
D) Not enough information is given.
Correct Answer:
Verified
Q6: Flux is defined as the rate at
Q7: What is the main difference between energy
Q8: A chiral center is an atom with
A)
Q9: Metabolism is best defined as a collection
Q10: How can an unfavorable reaction (
Q12: Which of the following pathways are found
Q13: In the following series of reactions,what is
Q14: Which of the following is an energy
Q15: Is the net reaction favorable for the
Q16: Glucose and fructose are both C6H12O6.What is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents