Aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorbent, desiccant, or catalyst for organic reactions. 4Al(s) + 3O2(g) 2Al2O3(s)
A mixture of 82.49 g of aluminum ( 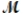 = 26.98 g/mol) and 117.65 g of oxygen (
= 26.98 g/mol) and 117.65 g of oxygen ( 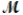 = 32.00 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
= 32.00 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
A) Oxygen is the limiting reactant; 19.81 g of aluminum remain.
B) Oxygen is the limiting reactant; 35.16 g of aluminum remain.
C) Aluminum is the limiting reactant; 16.70 g of oxygen remain.
D) Aluminum is the limiting reactant; 35.16 g of oxygen remain.
E) Aluminum is the limiting reactant; 44.24 g of oxygen remain.
Correct Answer:
Verified
Q45: Aluminum will react with bromine to
Q46: Phosphine, an extremely poisonous and highly
Q47: Ammonia, an important source of fixed
Q48: Aluminum oxide (used as an adsorbent
Q51: Lead(II) sulfide was once used in glazing
Q51: The iodine "clock reaction" involves the
Q52: Balance the following equation for the
Q53: How many grams of sodium fluoride
Q54: Balance the following equation:
Ca3(PO4)2(s) +
Q55: Aluminum metal reacts with chlorine gas to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents