How many milliliters of 1.58 M HCl are needed to react completely with 23.2 g of NaHCO3 ( 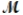 = 84.02 g/mol) ? HCl(aq) + NaHCO3(s) NaCl(s) + H2O(l) + CO2(g)
= 84.02 g/mol) ? HCl(aq) + NaHCO3(s) NaCl(s) + H2O(l) + CO2(g)
A) 638 mL
B) 572 mL
C) 536 mL
D) 276 mL
E) 175 mL
Correct Answer:
Verified
Q43: Which of the following is a strong
Q51: Select the net ionic equation for
Q53: Which, if any, of the following properties
Q57: Select the correct set of products
Q59: Calculate the oxidation number of sulfur in
Q62: In a redox reaction, electrons are transferred
A)from
Q64: A standard solution of 0.243 M NaOH
Q68: Vinegar is a solution of acetic acid,
Q70: Which of the following is a weak
Q74: Which one of the following substances is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents