Which of the lines on the figure below is the best representation of the relationship between the volume and the number of moles of a gas, measured at constant temperature and pressure? 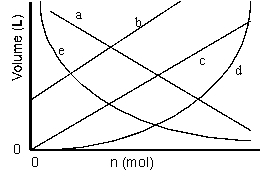
Correct Answer:
Verified
Q9: Mercury is 13.6 times as dense as
Q18: Given that pressure has dimensions of force
Q19: Mineral oil can be used in place
Q22: A sample of the inert gas krypton
Q28: The pressure of sulfur dioxide in a
Q33: A sample of nitrogen gas at 298
Q37: A sample of ammonia gas at 65.5°C
Q43: A sample of propane, a component of
Q46: A sample of methane gas, CH4(g), occupies
Q58: A sample container of carbon monoxide occupies
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents