Calculate the lattice energy of magnesium sulfide from the data given below.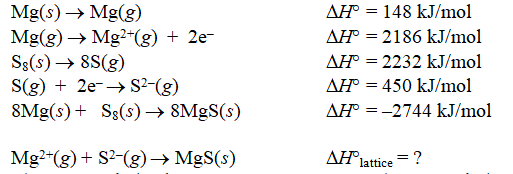
A) -3406 kJ/mol
B) -2720. kJ/mol
C) 2720. kJ/mol
D)3406 kJ/mol
E)none of the above
Correct Answer:
Verified
Q1: For which of the following elements (in
Q2: Which of the following is a covalent
Q14: In which of these substances are the
Q17: Which of the following is an ionic
Q18: The lattice energy of MgCl2 is
Q21: Arrange calcium, rubidium, sulfur, and arsenic in
Q23: Arrange aluminum, nitrogen, phosphorus, and indium in
Q30: Analysis of an unknown substance showed that
Q41: Based on electronegativity trends in the periodic
Q47: Combustion of a fat will release more
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents