Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ) the standard enthalpy change H° for the hydrogenation of ethyne (acetylene) to ethane. 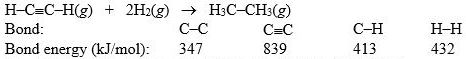
A) -296 kJ
B) -51 kJ
C) 51 kJ
D) 296 kJ
E) 381 kJ
Correct Answer:
Verified
Q21: Arrange calcium, rubidium, sulfur, and arsenic in
Q22: When two atoms form a covalently-bonded diatomic
Q23: Arrange aluminum, nitrogen, phosphorus, and indium in
Q26: Quartz (SiO2) is a solid with a
Q28: Arrange the following bonds in order of
Q29: Which one of the following properties is
Q31: Nitrogen and hydrogen combine to form
Q41: Based on electronegativity trends in the periodic
Q45: Which of the following elements is the
Q47: Combustion of a fat will release more
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents