Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon-oxygen double bond. Calculate the enthalpy of reaction using the bond energies given. 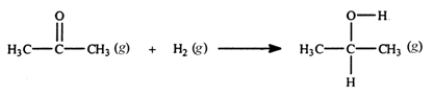

A) -484 kJ
B) -366 kJ
C) -48 kJ
D) +48 kJ
E) +366 kJ
Correct Answer:
Verified
Q23: Which one of the following properties is
Q26: Quartz (SiO2) is a solid with a
Q28: Arrange the following bonds in order of
Q29: Which one of the following properties is
Q31: Nitrogen and hydrogen combine to form
Q34: Select the strongest bond in the
Q37: Using the bond energies provided below,
Q38: Arrange oxygen, sulfur, calcium, rubidium, and potassium
Q52: Electronegativity is a measure of
A)the energy needed
Q57: Which of the following elements is the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents