The lattice energy of rubidium chloride is the energy change accompanying the process
Rb+(g) + Cl-(g) RbCl(s)
Calculate the lattice energy of RbCl using the following data: 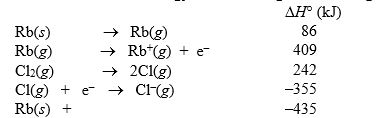
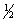 Cl2(g) RbCl(s)
Cl2(g) RbCl(s)
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q46: Select the most polar bond amongst the
Q49: In not more than three sentences, describe
Q50: When an atom is represented in a
Q51: Describe, with appropriate explanations, the key factors
Q52: Describe in brief how electronegativity values can
Q53: Using appropriate, real examples to illustrate your
Q55: In not more than three sentences, describe
Q56: Using appropriate, real examples to illustrate your
Q59: Which one of the following properties is
Q59: In not more than three sentences, describe
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents