Consider the phase diagram shown below. 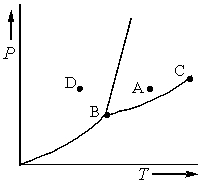 a. What phase(s) is/are present at point A?
a. What phase(s) is/are present at point A?
b. What phase(s) is/are present at point B?
c. Name point C and explain its significance.
d. Starting at D, if the pressure is lowered while the temperature remains constant, describe what will happen.
Correct Answer:
Verified
b. solid, liquid, and gas
c. C...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q64: The vapor pressure of 1-butene is 1.268
Q67: What word best describes the type of
Q68: The coordination number of sodium and chloride
Q73: In an ionic solid MX consisting of
Q81: The highest temperature at which superconductivity has
Q84: The energy gap between the conduction band
Q85: Which of the following statements about ceramics
Q87: For the solid forms of the following
Q88: When silicon is doped with an element
Q90: Which one of the following substances does
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents