In the gas phase at 500.°C, cyclopropane reacts to form propene in a first-order reaction. The figure below shows the concentration of cyclopropane plotted versus time. Use the graph to calculate approximate values of
a. the rate of the reaction, 600. seconds after the start.
b. the half-life of the reaction, t1/2. 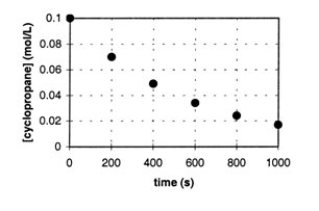
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q28: The decomposition of hydrogen peroxide is a
Q39: The rate law for the rearrangement of
Q40: The rate constant for the reaction
Q41: A rate constant obeys the Arrhenius equation,
Q44: Consider the following mechanism for the
Q46: If the activation energy of a reaction
Q61: The decomposition of dinitrogen pentaoxide to nitrogen
Q64: The kinetics of the decomposition of dinitrogen
Q66: An increase in temperature increases the reaction
Q71: A catalyst accelerates a reaction because
A)it increases
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents