In the gas phase at 500.°C, cyclopropane reacts to form propene in a first-order reaction. The figure shows the natural logarithm of the concentration of cyclopropane (in mol/L) plotted versus time. 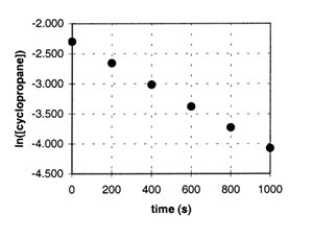 a. Explain how this plot confirms that the reaction is first order.
a. Explain how this plot confirms that the reaction is first order.
b. Calculate the first-order rate constant, k.
c. Determine the initial concentration of
cyclopropane in this experiment.
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q53: The gas-phase reaction CH3NC
Q54: The decomposition of dinitrogen pentaoxide has
Q55: Consider the following mechanism for the
Q57: When a catalyst is added to a
Q58: A boiled egg can be cooked at
Q58: A reaction has an activation energy of
Q59: You are studying the rate of
Q61: Briefly list the features/properties common to all
Q70: Reaction intermediates differ from activated complexes in
Q76: In an exothermic reaction,
A)the forward reaction is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents