Cyclobutane decomposes to ethene in a first-order reaction. From measurements of the rate constant (k) at various absolute temperatures (T), the accompanying Arrhenius plot was obtained (ln k versus 1/T). 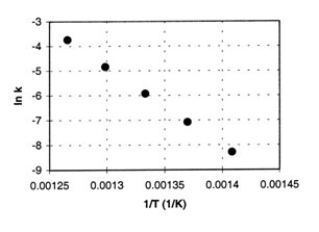 a. Calculate the energy of activation, Ea.
a. Calculate the energy of activation, Ea.
b. Determine the value of the rate constant at 740. K. (In the plot, the units of k are s-1.)
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: A transition state is a species (or
Q2: An elementary reaction is a simple, one-step
Q4: All second-order reactions are bimolecular reactions.
Q14: The units of the rate constant depend
Q71: Briefly outline the key arguments in
Q72: The elementary reaction HBr(g) + Br(g)
Q73: Is a bimolecular reaction necessarily second-order? Is
Q74: A chemical reaction of the general
Q75: You are required to determine the energy
Q78: The gas-phase conversion of 1,3-butadiene to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents