A diprotic acid H2A has Ka1 = 1 × 10-4 and Ka2 = 1 × 10-8. The corresponding base A2- is titrated with aqueous HCl, both solutions being 0.1 mol L-1. Which one of the following diagrams best represents the titration curve which will be seen?
A) 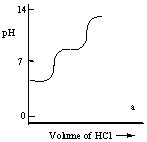
B) 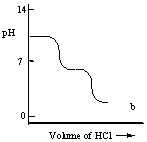
C) 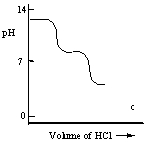
D) 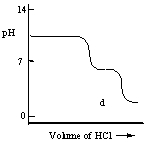
E) 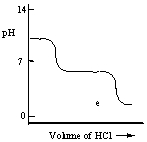
Correct Answer:
Verified
Q54: A 50.0-mL sample of 0.50 M HCl
Q56: Which one of the following is the
Q57: A 25.0-mL sample of 0.10 M C2H3NH2
Q59: Which one of the following is the
Q62: A saturated solution of calcium hydroxide, Ca(OH)2,
Q63: Write the ion product expression for magnesium
Q64: A change in pH will significantly affect
Q70: When 20.0 mL of 0.15 M hydrochloric
Q75: Calculate the solubility of barium carbonate, BaCO3,
Q96: Which of the following substances has the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents