You are given pure samples of pentane, CH3CH2CH2CH2CH3(l) , and 1,3-pentadiene, CH2=CHCH=CHCH3(l) . What prediction would you make concerning their standard molar entropies at 298 K?
A) S°pentane > S°1, 3-pentadiene
B) S°pentane < S°1, 3-pentadiene
C) S°pentane S°1, 3-pentadiene
D) S°pentane = S°1, 3-pentadiene + 2 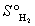
E) More information is needed to make reasonable predictions.
Correct Answer:
Verified
Q22: In which one of these pairs will
Q24: Which of the following pairs has the
Q27: You are given pure samples of
Q28: Elemental boron can be formed by
Q29: You are given pure samples of
Q30: For a chemical reaction to be
Q33: For a chemical reaction to be
Q35: A sample of water is heated at
Q35: For a chemical reaction to be
Q36: Calculate
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents