The temperature at which the following process reaches equilibrium at 1.0 atm is the normal boiling point of hydrogen peroxide. 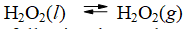
Use the following thermodynamic information at 298 K to determine this temperature. 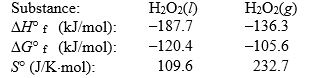
A) 120°C
B) 144°C
C) 196°C
D) 418°C
E) 585°C
Correct Answer:
Verified
Q38: Calculate
Q40: Calculate
Q40: In which one of the following pairs
Q41: Use the given data at 298
Q43: Consider the following quantities used in thermodynamics:
Q44: Sulfuryl dichloride is formed when sulfur
Q45: Hydrogen sulfide decomposes according to the
Q46: The temperature at which the following process
Q47: For a process with
Q48: "A diamond is forever" is one
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents