Iron(III) oxide can be reduced by carbon monoxide. 
Use the following thermodynamic data at 298 K to determine the equilibrium constant at this temperature. 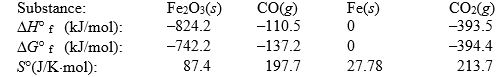
A) 7.0 × 10-6
B) 1.3 × 10-3
C) 2.2 × 104
D) 1.4 × 105
E) > 2.0 × 105
Correct Answer:
Verified
Q68: A reaction has
Q69: In the expression, S = k ln
Q70: The reaction of methane with water to
Q71: Consider the figure which shows
Q72: Consider the reaction
11ec6d53_97e1_49fd_9cd1_030bf8888a28_TB5833_
If the concentrations
Q74: For the reaction of xenon and
Q75: In tables of thermodynamic data provided
Q76: Consider the figure which shows
Q77: The formation constant for the reaction
Q78: A chemical reaction has
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents