Calculate the equilibrium constant at 25°C for the reaction of methane with water to form carbon dioxide and hydrogen. The data refer to 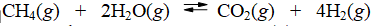
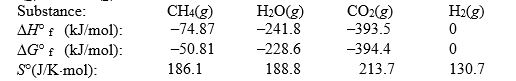
A) 8.2 × 1019
B) 0.96
C) 0.58
D) 1.2 × 10-20
E) 1.4 × 10-46
Correct Answer:
Verified
Q6: Under a given set of conditions, all
Q11: In some spontaneous processes, the entropy of
Q12: The term microstate refers to the energy
Q75: In tables of thermodynamic data provided
Q76: Consider the figure which shows
Q77: The formation constant for the reaction
Q78: A chemical reaction has
Q79: State the second and third laws of
Q81: For a given reaction, a change
Q83: A reaction has a positive value
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents