Examine the following half-reactions and select the weakest oxidizing agent among the species listed. 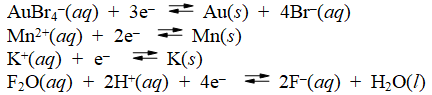
A) AuBr4-(aq)
B) Mn2+(aq)
C) K+(aq)
D) F2O(aq)
E) H+(aq)
Correct Answer:
Verified
Q29: A cell can be prepared from copper
Q29: The line notation, Al(s) | Al3+(aq) ||
Q30: What is the E°cell for the cell
Q31: What is the E°cell for the cell
Q32: Calculate E°cell and indicate whether the
Q34: Calculate E°cell and indicate whether
Q35: When metal A is placed in
Q36: The redox reaction of peroxydisulfate with
Q37: Examine the following half-reactions and select the
Q50: A battery is considered "dead" when
A)Q <
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents