Consider the reaction of iodine with manganese dioxide 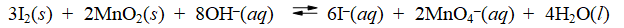
The equilibrium constant for the overall reaction is 8.30 × 10-7. Calculate G° for the reaction at 25°C.
A) -15.1 kJ
B) -34.7 kJ
C) 15.1 kJ
D) 34.7 kJ
E) none of the above
Correct Answer:
Verified
Q29: The line notation, Al(s) | Al3+(aq) ||
Q36: The redox reaction of peroxydisulfate with
Q37: Examine the following half-reactions and select the
Q40: Given that E° for X +
Q42: The value of the equilibrium constant
Q44: Calculate E°cell for the reaction of
Q53: Calculate the potential of a voltaic cell
Q73: Which, if any, of the following metals
Q78: A voltaic cell consists of an Au/Au3+
Q97: Which, if any, of the following metals
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents