Which one of the following is not a correct formation reaction? (products are correct)
A) H2(g) + O(g) H2O(l)
B) 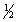 H2(g) +
H2(g) + 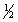 Cl2(g) HCl(g)
Cl2(g) HCl(g)
C) 6C(graphite) + 3H2(g) C6H6(l)
D) C(graphite) C(diamond)
E) 6C(graphite) + 6H2(g) + 3O2(g) C6H12O6(s)
Correct Answer:
Verified
Q7: For all processes, both q and w
Q14: The only way in which a system
Q55: Which one of the following is
Q56: Stoichiometric amounts of nitrogen gas and
Q57: The highly exothermic thermite reaction, in
Q58: Nitric acid, which is among the
Q59: Calculate the
Q61: For a reaction in a sealed,
Q64: Clearly state the thermodynamic standard state of
a.
Q65: Although internal energy (E) is more
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents