Which Reaction Has a Positive G°, Assuming That Entropy Changes Are Negligible Compared to Enthalpy
Which reaction has a positive G°, assuming that entropy changes are negligible compared to enthalpy changes? 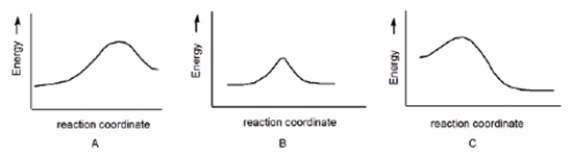
A) A
B) B
C) C
Correct Answer:
Verified
Q28: The equilibrium constant for the conversion of
Q29: Which reaction is slowest? Q31: How many transition states are present in Q33: Which step would most likely have the Q34: If the conversion of A to B Q35: Which of the following statements is true? Q36: Which of the following letters represents Q37: A decrease in which of the following Q37: In which reaction is Keq > 1? Q40: Which of the following statements is true?![]()
A)
A)Fast
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents