The stereoselective introduction of the C15 OH group on the side chain of prostaglandins has presented a challenge for synthetic chemists. Recently, Mulzer and co-workers demonstrated a new route in which the side chain is synthesized independently and then attached to the bicyclic counterpart. What is the appropriate sequence of reagents for the following synthesis? 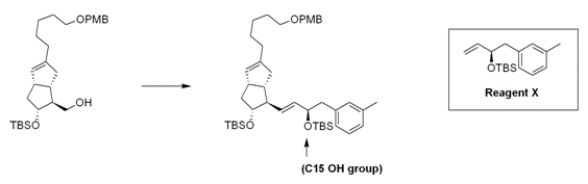
A) (1) PBr3; (2) Grubbs' catalyst, Reagent X; (3) KOC(CH3) 3
B) (1) PCC; (2) Ph3P=CH2; (3) Grubbs' catalyst, Reagent X
C) (1) PBr3; (2) Ph3P=CH2; (3) Grubbs' catalyst, Reagent X
D) (1) TsCl, pyridine; (2) Grubbs' catalyst, Reagent X; (3) KOC(CH3) 3
Correct Answer:
Verified
Q22: Which of the following sequences shows a
Q24: Triacylglycerol A yields compound B when treated
Q24: From the statements below,pick out the one
Q25: Which of the following fatty acids has
Q26: Which of the following is not one
Q28:
-Norethindrone is a synthetic steroid that is
Q29: Which of the following are complex lipids?
A)
Q30: Sphingomyelins are a major component of the
Q31: Patchouli alcohol is an example of a
Q40: What is the result of the oxidation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents