Treatment of 2-hexanone with NaOCH2CH3 followed by CH3Br affords compound X (C7H14O) as the major product. X shows a strong absorption in the IR spectrum at 1713 cm-1, and its 1H NMR data is given below. What is the structure of X? 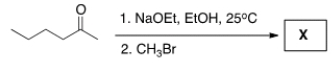
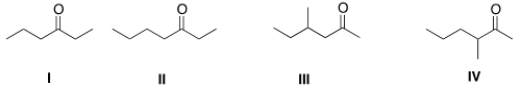
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q26: A simple chemical test to distinguish between
Q31: Which is the thermodynamic enolate of 2-methylcyclohexanone?
Q32: Which of the following ketones will give
Q32: The malonic ester synthesis can be
Q33: Which is the most acidic proton in
Q34: What is the starting material in the
Q35: It has been found that
Q38: Which is the most acidic proton in
Q39: What is the major product of the
Q41: What is the starting material required to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents