Why would the alcohol in the following compound need to be protected before reaction? 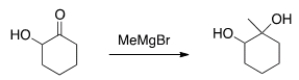
A) If it isn't protected, the product will be a carboxylic acid.
B) The Grignard reagent will react with the alcohol before the ketone.
C) Magnesium is Lewis acidic and will coordinate with the alcohol.
D) There is no need to protect the alcohol.
Correct Answer:
Verified
Q10: Which of the following terms explain why
Q17: Which reagent can be used to reduce
Q17: What is the starting material in the
Q19: What is the product of the following
Q20: What reagent would be used to reduce
Q21: What is the missing reagent in the
Q23: What is the major organic product of
Q24: What is the major organic product of
Q25: What is the major organic product of
Q32: What reagent can be used to cleave
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents