The base peak in a mass spectrum corresponds to the most stable fragment. Propose a structure for a compound that is consistent with the following data.
(a) The molecular ion peak has m/z = 116
(b) The base peak is at m/z = 59.
(c) The compound is composed of C, H and O atoms.
(d) The IR spectrum shows a strong absorbance at 3257 cm-1. 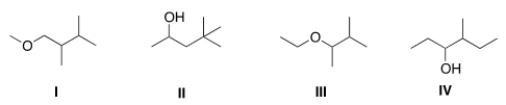
A) I
B) II
C) III
D) IV
Correct Answer:
Verified
Q5: Which of the following statement(s)is (are)true about
Q8: In a typical mass spectrum,a smaller signal
Q9: An alkyne C-H bond absorbs at higher
Q18: Why does an alkyne carbon-carbon triple bond
Q19: Compared to a C-H bond,a C-D bond
Q21: Examine the IR below and classify the
Q24: Examine the IR below and classify the
Q25: What type of signal(s) would you observe
Q26: Examine the IR below and classify the
Q27: When the phenol shown below is treated
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents