Determine the electron geometry around the indicated atom in each species. 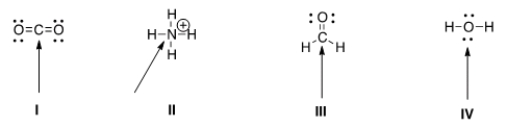
A) I = Linear; II = tetrahedral; III = trigonal planar; IV = tetrahedral
B) I = Linear; II = tetrahedral; III = trigonal planar; IV = linear
C) I = Trigonal planar; II = linear; III = tetrahedral; IV = trigonal planar
D) I = Tetrahedral; II = trigonal planar; III = linear; IV = tetrahedral
Correct Answer:
Verified
Q15: What is the ground-state electronic configuration of
Q19: What is the ground-state electronic configuration of
Q21: Which of the following is a resonance
Q22: How many constitutional isomers are there for
Q26: How many constitutional isomers are there for
Q27: Follow the curved arrows to draw the
Q37: How many different isomers are there for
Q43: What is the approximate H-C-O bond angle
Q49: What is the approximate bond angle for
Q56: What is the approximate C-C-C bond angle
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents